Understanding Calcined Petroleum Coke: Its Importance and Applications
Summary:
Calcined petroleum coke (CPC) is a highly valued material derived from the carbonization of petroleum products. It is produced by heating green petroleum coke in a rotary kiln at high temperatures, typically between 1200°C and 1400°C, in the absence of air. This calcination process removes volatile compounds and impurities, resulting in a product with a high carbon content, low sulfur levels, and

Calcined petroleum coke (CPC) is a highly valued material derived from the carbonization of petroleum products. It is produced by heating green petroleum coke in a rotary kiln at high temperatures, typically between 1200°C and 1400°C, in the absence of air. This calcination process removes volatile compounds and impurities, resulting in a product with a high carbon content, low sulfur levels, and excellent electrical conductivity. These characteristics make CPC an essential ingredient in various industrial processes.
One of the primary applications of calcined petroleum coke is in the aluminum industry. CPC is used as an anode material in the electrolytic production of aluminum. The high carbon content and conductivity of CPC allow for efficient electrolysis, which is essential for extracting aluminum from its ore. Additionally, the low sulfur content in CPC helps minimize the presence of impurities in the aluminum produced, ensuring higher quality products.
Another significant application of calcined petroleum coke is in the steel manufacturing process. In steel production, CPC serves as a carbon additive that enhances the carbon content of the steel, improving its hardness and strength. The use of CPC in steelmaking is becoming increasingly popular due to its cost-effectiveness and superior performance compared to traditional carbon sources.
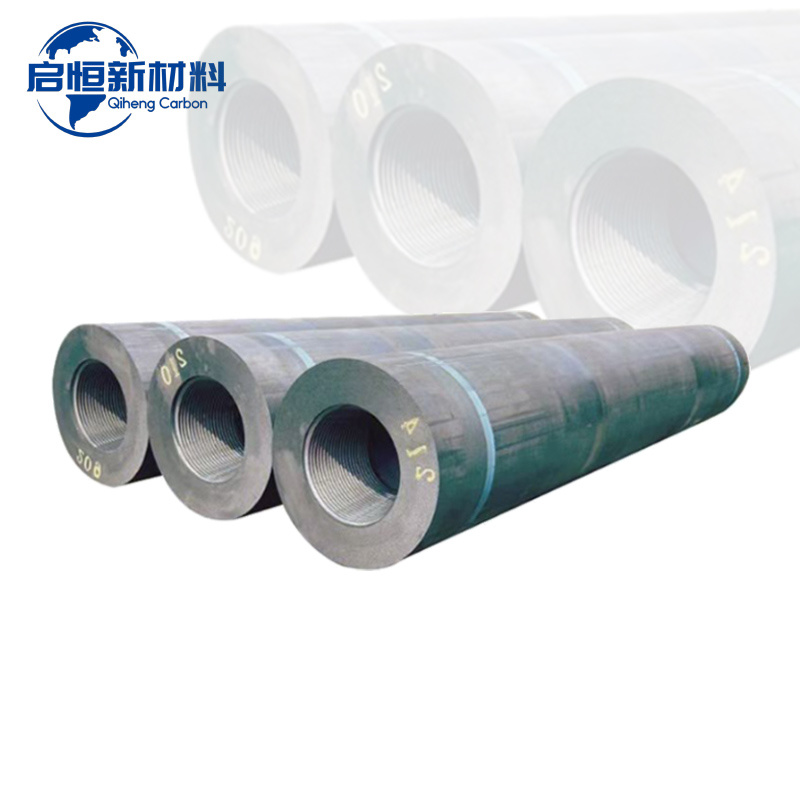
In addition to its use in aluminum and steel production, calcined petroleum coke is also utilized in the production of electrodes for electric arc furnaces. The high carbon content and conductivity of CPC make it an ideal material for manufacturing graphite electrodes, which are essential for various metallurgical processes. The demand for CPC in this application is driven by the increasing need for efficient and sustainable steel production methods.
The global market for calcined petroleum coke is influenced by factors such as the growth of the aluminum and steel industries, technological advancements in manufacturing processes, and increasing environmental regulations. As industries strive for more sustainable practices, the demand for high-quality, low-emission carbon products like CPC is expected to rise.
In conclusion, calcined petroleum coke is a vital material with diverse applications in the aluminum and steel industries. Its unique properties contribute to enhanced production efficiency and product quality, making it an indispensable resource for professionals in the chemical and petroleum sectors. Understanding the significance of CPC and its applications can provide valuable insights for strategic decision-making in these industries.
One of the primary applications of calcined petroleum coke is in the aluminum industry. CPC is used as an anode material in the electrolytic production of aluminum. The high carbon content and conductivity of CPC allow for efficient electrolysis, which is essential for extracting aluminum from its ore. Additionally, the low sulfur content in CPC helps minimize the presence of impurities in the aluminum produced, ensuring higher quality products.
Another significant application of calcined petroleum coke is in the steel manufacturing process. In steel production, CPC serves as a carbon additive that enhances the carbon content of the steel, improving its hardness and strength. The use of CPC in steelmaking is becoming increasingly popular due to its cost-effectiveness and superior performance compared to traditional carbon sources.
In addition to its use in aluminum and steel production, calcined petroleum coke is also utilized in the production of electrodes for electric arc furnaces. The high carbon content and conductivity of CPC make it an ideal material for manufacturing graphite electrodes, which are essential for various metallurgical processes. The demand for CPC in this application is driven by the increasing need for efficient and sustainable steel production methods.
The global market for calcined petroleum coke is influenced by factors such as the growth of the aluminum and steel industries, technological advancements in manufacturing processes, and increasing environmental regulations. As industries strive for more sustainable practices, the demand for high-quality, low-emission carbon products like CPC is expected to rise.
In conclusion, calcined petroleum coke is a vital material with diverse applications in the aluminum and steel industries. Its unique properties contribute to enhanced production efficiency and product quality, making it an indispensable resource for professionals in the chemical and petroleum sectors. Understanding the significance of CPC and its applications can provide valuable insights for strategic decision-making in these industries.
Previous:
Focus On Hot Spots
RP Graphite Electrodes: Transforming Conductivity in Metallurgical Processes
RP Graphite Electrodes: Enhancing Conductivity in Metallurgical Applications
Table of Contents
1. Introduction to RP Graphite Electrodes
2. Understanding Graphite and Its Properties
3. The Role of RP Graphite Electrodes in Metallurgy
4. Benefits of Using RP Graphite Electrodes
5. The Manufacturing Process of RP Graphite Electrodes
6. Applications of RP Graphite Electrodes
The Essential Guide to Graphite Blocks in Metallurgy and Energy Industries
Graphite blocks are pivotal materials in the metallurgy and energy industries, particularly within the non-metallic mineral products sector. These blocks, made from natural or synthetic graphite, possess unique properties that make them suitable for a wide range of applications. One of the most notable characteristics of graphite is its excellent thermal and electrical conductivity. This property